Dissolved Oxygen
Overview
Dissolved oxygen (DO) is the amount of oxygen present in the water. Just like humans, all of the Chesapeake Bay's living creatures—from the fish and crabs that swim through its waters to the worms that bury themselves in its mud—need oxygen to survive. While humans use their lungs to inhale oxygen from the air, aquatic animals use their gills to absorb oxygen from the water. As water moves across an animal’s gills, oxygen passes into the animal’s bloodstream.
Watch: Bay 101: Dissolved Oxygen
How does oxygen get into the water?
Oxygen enters the Bay in several ways:
- Oxygen from the atmosphere dissolves and mixes into the water’s surface.
- Algae and underwater grasses release oxygen during photosynthesis.
- Water flows into the Bay from streams, rivers and the ocean. Ocean waters generally contain more oxygen. River waters are fast-moving, which helps oxygen from the air mix in.
How much oxygen do aquatic animals need?
Dissolved oxygen is measured in milligrams of oxygen per liter of water (mg/L). Scientists generally agree that the Bay’s critters need dissolved oxygen concentrations of 5 mg/L or more to thrive. However, the amount of oxygen an animal needs depends on its size and habitat.
- Worms and clams that live in the Bay's muddy bottom—where oxygen levels are naturally low—need dissolved oxygen concentrations of at least 1 mg/L.
- Fish, crabs, and oysters that live or feed along the bottom require dissolved oxygen concentrations of 3 mg/L or more.
- Spawning migratory fish and their eggs and larvae need up to 6 mg/L during these sensitive life stages.
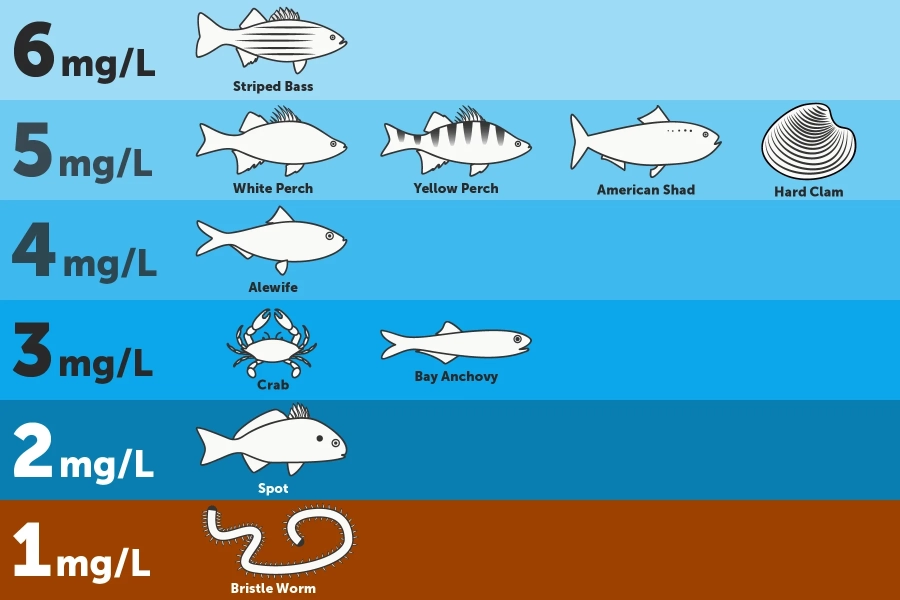
Show image description
The infographic divides the Chesapeake Bay's water column into six levels, based on depth. The infographic includes drawings of different animals at different depths, next to the minimum amount of oxygen those animals need to survive. On the Bay's bottom, where a bristle worm is shown, species need dissolved oxygen concentrations of at least 1mg/L. Above that, where a fish known as a spot is shown, species need dissolved oxygen concentrations of at least 2 mg/L. Above that, where a crab and bay anchovy are shown, species need dissolved oxygen concentrations of at least 3mg/L. Above that, where an alewife is shown, species need dissolved oxygen concentrations of at least 4mg/L. Above that, where a white perch, yellow perch, American shad, and hard clam are shown, species need dissolved oxygen concentrations of at least 5mg/L. And at the top of the Bay's water column, where a striped bass is shown, species need dissolved oxygen concentrations of at least 6mg/L.
What is a dead zone?
Dead zones are areas with little to no dissolved oxygen. Low-oxygen or hypoxic areas are regions with less than 2 mg/L of dissolved oxygen. No-oxygen or anoxic areas are regions with less than 0.2 mg/L of dissolved oxygen.
Plants and animals are unable to survive low-oxygen environments, and dead zones are often the cause of fish kills, or the sudden death of large numbers of fish. While marine species like fish and crabs can move away from low-oxygen areas, species like oysters, whose habitat cannot be moved, are particularly vulnerable to dead zones.
How do dead zones form?
Dead zones are the result of several natural and man-made factors, including water temperature, nutrient pollution, water flow, and the shape of the Bay's bottom.
High temperatures
Temperature limits the amount of oxygen that can dissolve in water: water can hold more dissolved oxygen during cold months than hot months. However, temperature is not the only cause of the low-oxygen areas found in the Bay each summer. Even at the Bay’s warmest temperatures (around 91 degrees Fahrenheit), water is capable of having dissolved oxygen concentrations of 6 to 7 mg/L.
Nutrient pollution
Excess nutrients in the water can fuel the growth of algae blooms. While oysters, menhaden and other filter feeders eat some of the excess algae, much of it is left to die and sink to the Bay’s bottom. There, the algae is decomposed by bacteria that consume oxygen until there is little or none left.
Flow of water
The difference between water flowing into the Bay from the ocean and water flowing into the Bay from its rivers and streams can also influence dissolved oxygen levels. Ocean water is generally cool and salty, while river water is warm and fresh. River water is also less dense than ocean water, and generally floats on top.
During warm summer months, when algae blooms are more likely to form and algae-consuming bacteria are most active, the stratification between warm, light fresh water and cold, dense saltwater is strong. The barrier between oxygen-rich surface waters and oxygen-deprived bottom waters can create large areas of low- or no-oxygen at the bottom of the Bay. This problem often occurs in certain bowl-shaped areas along the Bay’s bottom, where deep water is effectively cut off from receiving any oxygen.
Learn more about the flow of water
Watch: Bay 101: Algae Blooms
Do scientists measure dead zones?
From May through October, researchers with the Maryland Department of Natural Resources and Virginia Department of Environmental Quality conduct monitoring cruises to keep an eye on the Bay’s dead zones. Data collected in Maryland waters are posted on Eyes on the Bay. Data collected in Virginia waters are posted on the Virginia Estuarine and Coastal Observing System website. Scientists at the Virginia Institute of Marine Science (VIMS) also produce daily forecasts of dissolved oxygen levels across the Bay.
What’s being done to limit dead zones?
Reducing the amount of nutrient pollution entering the Bay will reduce the size and duration of summer dead zones. While nutrients are a natural part of the Bay ecosystem, excess nutrients enter the water from wastewater treatment plants; urban, suburban and agricultural runoff; and air pollution. Pollution reducing practices can help reduce nutrient runoff.
